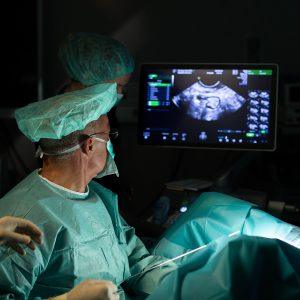
A New Path to Fertility
Ovarian revitalization methods have gained popularity worldwide, offering hope to women looking to improve their hormonal balance and reproductive potential. One of the most widely used approaches is hcPRP (highly concentrated platelet-rich plasma) therapy, which harnesses the power of the body’s own growth factors to stimulate ovarian function.
What is hc PRP Therapy?
Hc PRP is derived from the patient’s blood and contains powerful growth factors that promote cell growth, tissue repair, and hormone production. These factors play a crucial role in egg development and overall ovarian health.
How Does It Work?
Plasma is rich in platelets, which contain numerous growth factors responsible for healing and regeneration. In hc PRP, the platelet concentration is significantly higher than in regular plasma, allowing for enhanced activation of ovarian tissue. When injected into the ovaries, these growth factors stimulate follicle growth and hormone production naturally.
Autologous Bioregenerative Fibrin (BRF) as a Biological Scaffold
In combination with hc PRP, autologous bioregenerative fibrin (BRF) is used as a biological scaffold to enhance the effects of ovarian rejuvenation. BRF provides a supportive matrix that helps retain and gradually release growth factors at the injection site, promoting prolonged tissue repair and regeneration. This combination offers a synergistic effect, improving the structural integrity of ovarian tissue and enhancing the overall efficacy of the treatment.
Why hc PRP is Effective
Advanced processing techniques in the SEGOVA hc PRP program allow for a higher concentration of growth factors compared to standard methods. Special technology can increase cell concentration up to 18 times, providing personalized treatment tailored to each patient’s needs.
Key Benefits of hc PRP Ovarian Rejuvenation:
- Enhances egg quality and ovarian function.
- Uses the body’s own natural healing processes.
- Minimally invasive with minimal downtime.
What to Expect from Treatment
The procedure is quick and generally well-tolerated. Full benefits can be observed within a few months, with improved hormonal levels and ovarian response. For those aiming to conceive, hc PRP therapy can be combined with fertility treatments such as IVF.
Safety and Consideration
As hcPRP uses the patient’s own blood, risks are minimal. The procedure has a low complication rate, with mild side effects such as temporary discomfort or slight bruising at the injection site.
HcPRP ovarian rejuvenation offers an exciting and natural way to support fertility and hormonal health, helping women regain their confidence and reproductive potential.